Neuropsychiatric systemic lupus erythematosus (NPSLE) represents one of the most complex and challenging manifestations of systemic lupus erythematosus, affecting the central and peripheral nervous systems in ways that can profoundly impact a patient’s quality of life and long-term prognosis. While the prevalence of neuropsychiatric symptoms in lupus patients ranges from 12% to 95% depending on the diagnostic criteria and methodologies employed, recent advances in our understanding of the underlying pathophysiology, diagnostic biomarkers, and therapeutic approaches have opened new avenues for more precise and personalized treatment strategies.
The complexity of NPSLE stems from its heterogeneous clinical presentation, which can manifest as focal or diffuse neurological symptoms ranging from subtle cognitive dysfunction to severe acute confusional states, seizures, psychosis, and cerebrovascular events. This diversity in clinical manifestations reflects the multifactorial pathogenesis of NPSLE, involving inflammatory cytokines, autoantibodies, immune complexes, and vascular pathology that collectively contribute to neuronal injury through vasculopathic, cytotoxic, and autoantibody-mediated mechanisms.
Pathophysiological Foundations of Neuropsychiatric Lupus
The pathogenesis of NPSLE involves a complex interplay between systemic inflammation, autoantibody production, and blood-brain barrier dysfunction. The central role of inflammatory cytokines, particularly interleukin-6, tumor necrosis factor-alpha, and interferon-alpha, extends beyond their peripheral effects to directly influence brain function through alteration of neurotransmitter pathways, particularly those involving serotonin, dopamine, and norepinephrine. These inflammatory mediators can cross the blood-brain barrier or induce local cytokine production within the central nervous system, leading to neuroinflammation that manifests as cognitive dysfunction, mood disorders, and behavioral changes.
Autoantibodies play a crucial role in NPSLE pathogenesis, with several antibody specificities demonstrating particular relevance to neuropsychiatric manifestations. Anti-ribosomal P antibodies, found in approximately 20-30% of SLE patients, show strong associations with psychosis and severe depression. These antibodies can bind to ribosomal P proteins present in both hepatocytes and neurons, potentially explaining their hepatotoxic and neurotoxic effects. Anti-N-methyl-D-aspartate receptor antibodies, while more commonly associated with paraneoplastic encephalitis, have been identified in subsets of NPSLE patients, particularly those presenting with seizures, cognitive dysfunction, and psychiatric symptoms.
The role of antiphospholipid antibodies in NPSLE extends beyond their well-established thrombotic effects. These antibodies can directly interact with neuronal membranes, affecting synaptic transmission and neuronal function independent of their prothrombotic properties. Anticardiolipin antibodies and lupus anticoagulant have been associated with cognitive dysfunction, mood disorders, and movement disorders in NPSLE patients, even in the absence of overt thrombotic events.
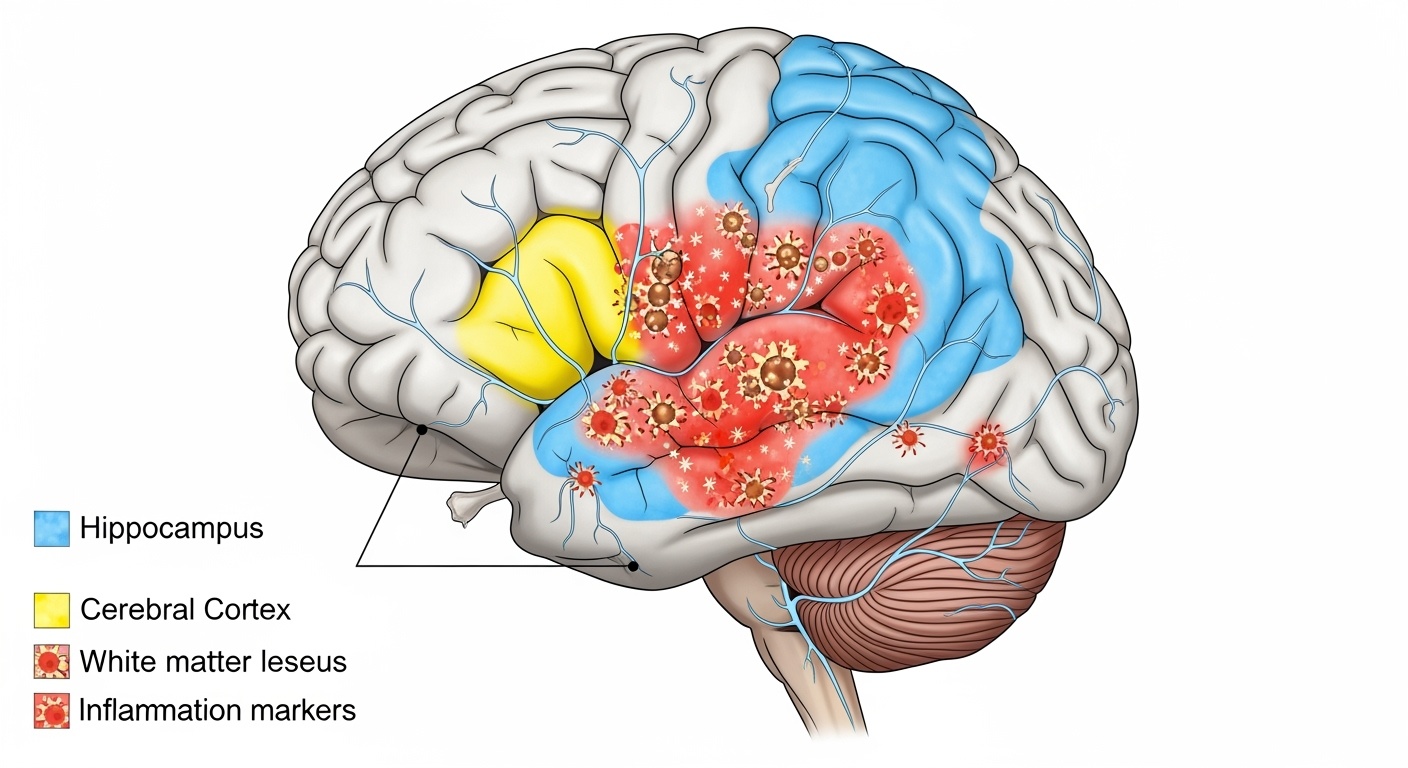
Complement activation represents another critical pathway in NPSLE pathogenesis. Complement components C3a and C5a can directly activate microglia and astrocytes, leading to neuroinflammation and synaptic pruning. Recent studies have identified elevated cerebrospinal fluid C3 levels as potential biomarkers for NPSLE, reflecting local complement activation within the central nervous system.
Clinical Spectrum and Phenotypic Heterogeneity
The clinical manifestations of NPSLE encompass a broad spectrum of neurological and psychiatric symptoms that can be categorized into focal and diffuse syndromes. Focal manifestations include seizures, which occur in approximately 15-20% of SLE patients, cerebrovascular disease affecting 5-15% of patients, and movement disorders including chorea, which affects 1-2% of SLE patients and may be the presenting feature of the disease.
Cognitive dysfunction represents the most common diffuse manifestation of NPSLE, affecting 80-90% of patients when assessed using comprehensive neuropsychological testing. The pattern of cognitive impairment typically involves multiple domains, including attention and concentration, processing speed, executive function, and memory consolidation. Unlike the global cognitive decline seen in neurodegenerative diseases, NPSLE-associated cognitive dysfunction often presents with a characteristic pattern of preserved general intelligence with specific deficits in working memory, psychomotor speed, and visuospatial processing.
Mood disorders, particularly depression and anxiety, occur in 30-60% of NPSLE patients, representing a complex interaction between disease-related inflammation, medication effects, and psychological adjustment to chronic illness. The presence of specific autoantibodies, particularly anti-ribosomal P antibodies, may help distinguish primary NPSLE-related mood disorders from reactive depression, as these antibodies show strong associations with severe depression and suicidal ideation.
Acute confusional states, occurring in 5-15% of SLE patients, can present as delirium, acute psychosis, or catatonia. These presentations often represent medical emergencies requiring prompt recognition and aggressive immunosuppressive treatment. The differential diagnosis includes medication-induced delirium, infectious complications, and metabolic encephalopathy, making careful clinical assessment and appropriate diagnostic testing essential.
Advanced Diagnostic Approaches and Biomarker Discovery
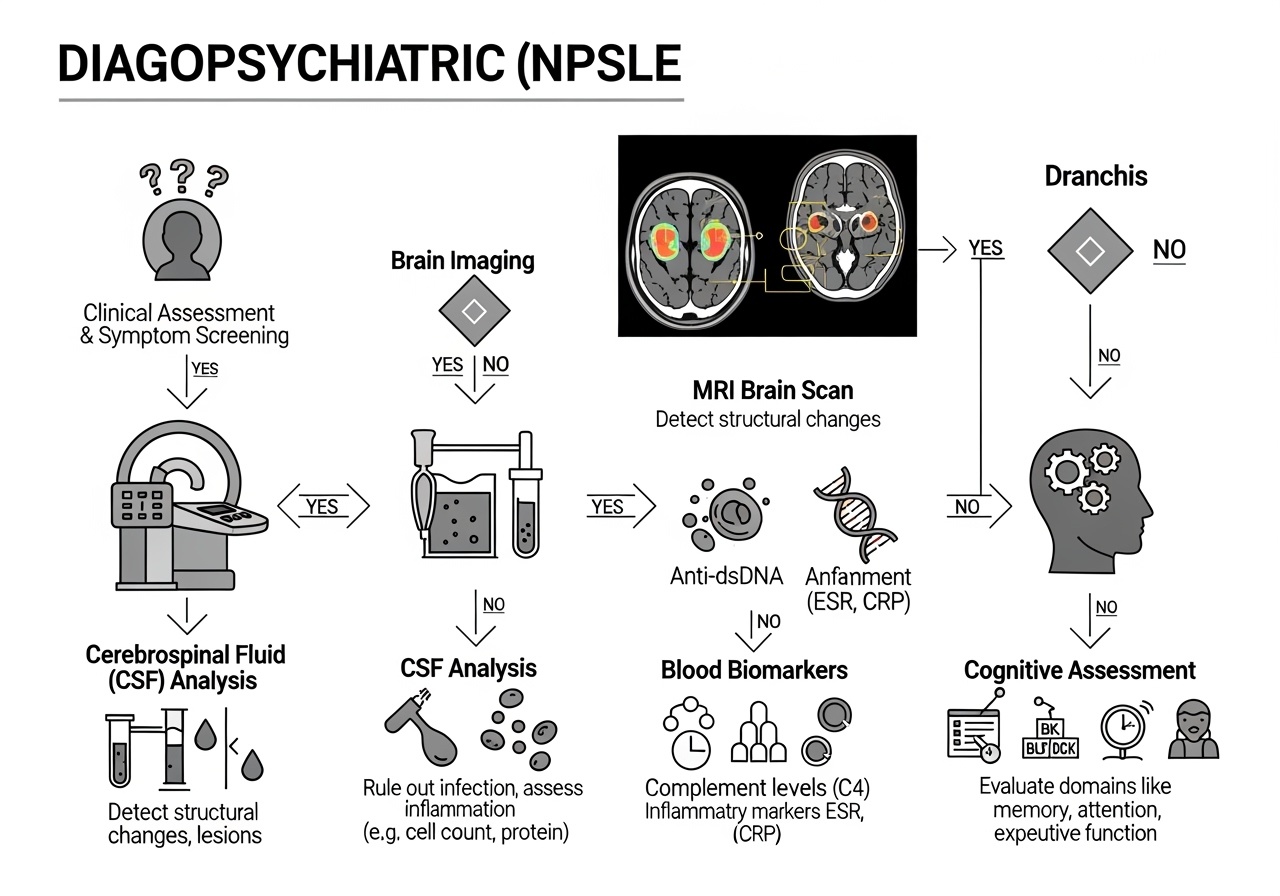
The diagnosis of NPSLE remains challenging due to the absence of pathognomonic clinical features or definitive diagnostic tests. However, recent advances in biomarker discovery and neuroimaging have significantly enhanced our diagnostic capabilities. The identification of novel cerebrospinal fluid biomarkers represents a major breakthrough in NPSLE diagnosis. Proteomic studies have identified several promising biomarkers, including kallikrein-5 (KLK5), L-selectin, Trappin-2, transcobalamin-2 (TCN2), and cystatin-6 (CST6), which demonstrate high diagnostic accuracy when combined in multivariate diagnostic models.
Serum neurofilament light chain has emerged as a valuable biomarker for neuronal damage in NPSLE, with elevated levels correlating with disease activity and cognitive dysfunction. This biomarker offers the advantage of being measurable in serum rather than cerebrospinal fluid, making it more accessible for routine clinical monitoring. Anti-microtubule-associated protein-2 antibodies in cerebrospinal fluid have shown particular promise as diagnostic biomarkers, demonstrating high specificity for NPSLE when present.
The development of autoantibody panels specific for NPSLE has enhanced diagnostic precision. Beyond traditional markers such as anti-ribosomal P and antiphospholipid antibodies, newer targets including anti-NMDA receptor antibodies, anti-aquaporin-4 antibodies, and anti-neuronal antibodies provide additional diagnostic information. The presence of multiple autoantibodies appears to correlate with more severe neuropsychiatric manifestations and may guide treatment intensity.
Neuroimaging advances have revolutionized NPSLE diagnosis and monitoring. Conventional magnetic resonance imaging reveals abnormalities in 50-80% of NPSLE patients, with patterns including white matter hyperintensities, cortical atrophy, and acute ischemic changes. Advanced imaging techniques such as diffusion tensor imaging can detect microstructural white matter changes even when conventional imaging appears normal. Functional magnetic resonance imaging studies have identified altered connectivity patterns in cognitive networks, providing insights into the neural basis of NPSLE-related cognitive dysfunction.
Magnetic resonance spectroscopy can detect metabolic changes in brain tissue, including elevated choline/creatine ratios suggesting membrane turnover and reduced N-acetylaspartate levels indicating neuronal dysfunction. These metabolic markers may precede structural changes visible on conventional imaging, offering potential for earlier diagnosis and intervention.
Cognitive Assessment and Neuropsychological Evaluation
Comprehensive cognitive assessment forms a cornerstone of NPSLE diagnosis and monitoring, requiring specialized neuropsychological testing that goes beyond standard mental status examinations. The American College of Rheumatology neuropsychological battery, developed specifically for SLE patients, includes validated tests covering six cognitive domains: simple attention, complex attention, memory, visuospatial processing, language, and executive function.
The battery includes specific tests such as the Trail Making Test for executive function and processing speed, the Rey Auditory Verbal Learning Test for memory assessment, and the Controlled Oral Word Association Test for language function. These tests have been validated in SLE populations and demonstrate sensitivity to the specific pattern of cognitive dysfunction seen in NPSLE.
Computerized cognitive assessment tools offer advantages in terms of standardization, accessibility, and longitudinal monitoring. The Automated Neuropsychological Assessment Metrics (ANAM) and similar platforms can provide rapid screening for cognitive dysfunction and track changes over time. However, these tools should complement rather than replace comprehensive neuropsychological evaluation when NPSLE is suspected.
The Montreal Cognitive Assessment (MoCA) has shown utility as a screening tool for cognitive dysfunction in SLE patients, demonstrating greater sensitivity than the Mini-Mental State Examination for detecting mild cognitive impairment. A cutoff score of 26 or below on the MoCA shows good sensitivity and specificity for identifying NPSLE-related cognitive dysfunction.
Performance validity testing has become increasingly important in NPSLE assessment, as patients may experience fatigue, depression, or other factors that can affect test performance independent of cognitive ability. Embedded validity indicators within neuropsychological tests help ensure that observed deficits reflect genuine cognitive impairment rather than suboptimal effort or other confounding factors.
Neuroimaging Patterns and Radiological Insights
Magnetic resonance imaging findings in NPSLE demonstrate characteristic patterns that can inform diagnosis and treatment decisions. White matter hyperintensities on T2-weighted and FLAIR sequences represent the most common abnormality, occurring in 60-80% of NPSLE patients. These lesions typically appear as small, punctate hyperintensities in periventricular and subcortical regions, though larger confluent lesions may occur in severe cases.
The distribution of white matter lesions in NPSLE differs from that seen in multiple sclerosis or small vessel disease. NPSLE lesions tend to have a more random distribution without the characteristic periventricular perpendicular orientation seen in multiple sclerosis. The presence of lesions in the corpus callosum, while not specific to NPSLE, may support the diagnosis when present in the appropriate clinical context.
Cerebral atrophy represents another important finding in NPSLE, occurring in up to 67% of patients and correlating with disease duration and cumulative steroid exposure. Both global and regional atrophy patterns have been described, with particular involvement of the hippocampus and frontal cortex corresponding to observed deficits in memory and executive function.
Acute cerebrovascular changes, including both ischemic and hemorrhagic lesions, occur in 5-15% of NPSLE patients. These may result from vasculitis, thromboembolism related to antiphospholipid antibodies, or hypertensive complications of treatment. The presence of multiple acute lesions in different vascular territories may suggest a systemic etiology such as NPSLE rather than traditional stroke mechanisms.
Advanced imaging techniques provide additional insights into NPSLE pathophysiology. Diffusion tensor imaging can detect microstructural white matter changes reflected in altered fractional anisotropy and mean diffusivity values. These changes may precede visible lesions on conventional imaging and correlate with cognitive dysfunction severity.
Perfusion imaging using arterial spin labeling or dynamic susceptibility contrast techniques can identify regions of altered cerebral blood flow. Hypoperfusion patterns may reflect inflammatory vasculopathy or complement-mediated microvascular dysfunction, while hyperperfusion may indicate acute inflammation or luxury perfusion following ischemic injury.
Personalized Treatment Strategies and Therapeutic Innovations
The management of NPSLE requires a personalized approach that considers the specific clinical phenotype, underlying pathophysiology, and individual patient factors. Treatment strategies must balance the need for aggressive immunosuppression to control neuroinflammation with the potential risks of therapy, particularly in patients with concurrent medical comorbidities.
Corticosteroids remain the foundation of acute NPSLE treatment, with high-dose pulse methylprednisolone (1000 mg daily for 3-5 days) often employed for severe manifestations such as acute confusional states, seizures, or cerebrovascular events. The rapid anti-inflammatory effects of corticosteroids can be life-saving in acute NPSLE, though their long-term use is limited by significant side effects including cognitive dysfunction, mood changes, and increased infection risk.
Cyclophosphamide has demonstrated efficacy in severe NPSLE, particularly for manifestations such as cerebritis, myelitis, and refractory seizures. The drug’s potent immunosuppressive effects target multiple pathogenic pathways in NPSLE, including autoantibody production, T-cell activation, and inflammatory cytokine release. Both monthly intravenous pulse therapy and daily oral administration have been employed, with the pulse regimen generally preferred due to reduced cumulative toxicity.
Mycophenolate mofetil represents an increasingly popular alternative to cyclophosphamide for NPSLE treatment, offering similar efficacy with potentially fewer side effects. Studies comparing mycophenolate mofetil to cyclophosphamide in NPSLE have shown comparable response rates with better tolerability profiles. The drug’s selective inhibition of lymphocyte proliferation makes it particularly suitable for patients requiring long-term immunosuppression.
Rituximab has emerged as a valuable treatment option for refractory NPSLE, demonstrating particular efficacy for acute confusional states and severe cognitive dysfunction. The drug’s mechanism of B-cell depletion addresses multiple pathogenic pathways including autoantibody production and antigen presentation. Case series and small studies have reported response rates of 70-80% in refractory NPSLE, with effects typically apparent within 2-4 weeks of treatment.
The personalization of rituximab therapy involves consideration of B-cell phenotyping and autoantibody profiles. Patients with high autoantibody titers or evidence of active B-cell activation may derive particular benefit from rituximab treatment. Combination protocols using rituximab with cyclophosphamide or mycophenolate mofetil are being investigated for patients with severe refractory disease.
Belimumab, a monoclonal antibody targeting B-lymphocyte stimulator (BLyS), represents a newer addition to the NPSLE therapeutic armamentarium. While not specifically approved for NPSLE, post-hoc analyses of clinical trials suggest potential benefits for neuropsychiatric manifestations. The drug’s mechanism of inhibiting B-cell survival and activation addresses key pathogenic pathways in NPSLE while potentially offering a more favorable side effect profile than traditional immunosuppressants.
| Treatment Category | Primary Agents | Mechanism of Action | Clinical Applications |
| Acute Therapy | Methylprednisolone | Anti-inflammatory, immunosuppressive | Severe acute manifestations, cerebritis |
| Conventional Immunosuppressants | Cyclophosphamide, Mycophenolate mofetil | Lymphocyte inhibition | Maintenance therapy, moderate-severe disease |
| Targeted Biologics | Rituximab, Belimumab | B-cell depletion/modulation | Refractory disease, specific autoantibody profiles |
Emerging Therapeutic Targets and Future Directions
The evolving understanding of NPSLE pathophysiology has identified several promising therapeutic targets for future drug development. Complement inhibition represents a particularly attractive target given the role of complement activation in NPSLE pathogenesis. Eculizumab, a monoclonal antibody targeting complement component C5, has shown promise in case reports of refractory NPSLE, though larger studies are needed to establish efficacy and safety.
Cytokine-targeted therapies offer another promising avenue for NPSLE treatment. Tocilizumab, an interleukin-6 receptor antagonist, has demonstrated efficacy in several autoimmune conditions and may have particular relevance for NPSLE given the role of IL-6 in neuroinflammation and cognitive dysfunction. Preliminary reports suggest potential benefits in refractory cases, though controlled studies are needed.
Janus kinase (JAK) inhibitors represent a novel class of targeted therapy with potential applications in NPSLE. These small-molecule inhibitors can modulate multiple cytokine signaling pathways simultaneously, potentially offering broader anti-inflammatory effects than single-target biologics. Tofacitinib and baricitinib are being investigated in SLE, with potential future applications in NPSLE.
Neuroprotective strategies are increasingly being explored as adjunctive treatments for NPSLE. N-acetylcysteine, with its antioxidant and glutamate-modulating properties, has shown promise in small studies for improving cognitive function in SLE patients. Memantine, an NMDA receptor antagonist, may have theoretical benefits given the role of anti-NMDA receptor antibodies in NPSLE pathogenesis.
The integration of precision medicine approaches into NPSLE treatment involves the use of biomarker-guided therapy selection. Patients with specific autoantibody profiles or cytokine signatures may benefit from targeted therapeutic approaches. For example, patients with anti-ribosomal P antibodies might benefit from treatments specifically targeting B-cell activation, while those with evidence of complement activation might respond better to complement inhibition.
| Biomarker Category | Specific Markers | Clinical Application | Therapeutic Implications |
| Autoantibodies | Anti-ribosomal P, Anti-NMDA receptor | Phenotype identification | Targeted B-cell therapies |
| Inflammatory Markers | CSF cytokines, Complement products | Disease monitoring | Anti-inflammatory intensity |
| Neuronal Damage | Neurofilament light chain | Prognosis assessment | Neuroprotective interventions |
Monitoring and Long-term Management Considerations
The long-term management of NPSLE requires systematic monitoring approaches that can detect disease activity changes and treatment responses while minimizing unnecessary interventions. Regular neuropsychological assessment provides objective measures of cognitive function that can guide treatment decisions and monitor therapeutic responses. The frequency of assessment should be tailored to disease activity and treatment intensity, with more frequent monitoring during active disease phases.
Biomarker monitoring offers the potential for more objective disease assessment than clinical measures alone. Serial measurement of cerebrospinal fluid or serum biomarkers can provide insights into ongoing neuroinflammation and treatment response. However, the invasive nature of cerebrospinal fluid sampling limits its routine use, making the development of reliable serum biomarkers a priority for clinical practice.
Neuroimaging surveillance plays an important role in NPSLE monitoring, particularly for patients with structural brain lesions or those at high risk for cerebrovascular complications. The optimal frequency and modality of imaging surveillance remain topics of ongoing research, with considerations including radiation exposure, cost-effectiveness, and clinical utility.
The management of treatment-related complications represents a significant challenge in NPSLE care. Corticosteroid-induced complications including cognitive dysfunction, mood changes, and increased infection risk require careful monitoring and mitigation strategies. Steroid-sparing immunosuppressive regimens should be employed whenever possible to minimize these risks while maintaining disease control.
Patient education and psychosocial support are integral components of comprehensive NPSLE care. Patients and families need education about the nature of neuropsychiatric symptoms, treatment expectations, and warning signs that should prompt immediate medical attention. Support groups and mental health resources can help patients cope with the psychological impact of cognitive dysfunction and other neuropsychiatric symptoms.
Interdisciplinary Care Models and Clinical Integration
The complexity of NPSLE necessitates interdisciplinary care models that integrate expertise from rheumatology, neurology, psychiatry, and neuropsychology. Effective care coordination requires clear communication channels between specialists and well-defined roles and responsibilities for each team member.
Rheumatologists typically serve as the primary coordinators of care, managing immunosuppressive therapies and monitoring systemic disease activity. Their expertise in SLE pathophysiology and treatment provides the foundation for NPSLE management decisions. However, the complexity of neuropsychiatric manifestations often requires input from neurological and psychiatric specialists.
Neurologists contribute expertise in the evaluation and management of seizures, movement disorders, and cerebrovascular complications of NPSLE. Their involvement is particularly important for patients with focal neurological deficits or those requiring specialized neurological interventions. Collaboration between rheumatology and neurology is essential for optimizing treatment regimens that address both systemic and neurological manifestations.
Psychiatrists play crucial roles in the evaluation and treatment of mood disorders, psychosis, and other psychiatric manifestations of NPSLE. Their expertise in psychopharmacology is valuable for managing symptoms while considering potential interactions with immunosuppressive medications. The distinction between primary psychiatric symptoms and NPSLE-related manifestations often requires specialized psychiatric evaluation.
Neuropsychologists provide essential services in cognitive assessment, rehabilitation, and monitoring. Their expertise in cognitive testing and interpretation is crucial for accurate diagnosis and treatment monitoring. Cognitive rehabilitation strategies can help patients develop coping mechanisms and optimize functional outcomes despite persistent cognitive deficits.
Future Research Directions and Clinical Translation
The future of NPSLE research lies in several key areas that promise to transform our understanding and treatment of this complex condition. Genomic and transcriptomic studies are beginning to identify genetic factors that predispose to NPSLE development and influence treatment response. These insights may lead to personalized treatment approaches based on individual genetic profiles.
Advanced neuroimaging techniques continue to evolve, offering new insights into NPSLE pathophysiology and potential therapeutic targets. Positron emission tomography imaging with specific tracers for neuroinflammation and microglial activation may provide more sensitive measures of disease activity than current methods.
The development of patient-reported outcome measures specific to NPSLE represents an important research priority. Current outcome measures often fail to capture the full impact of neuropsychiatric symptoms on patient quality of life and functional status. Disease-specific instruments could improve clinical trial design and routine clinical monitoring.
Clinical trial design for NPSLE faces unique challenges including heterogeneous clinical presentations, difficulty in outcome measurement, and small patient populations. Innovative trial designs including adaptive protocols, enrichment strategies, and biomarker-driven endpoints may facilitate more efficient evaluation of new therapeutic approaches.
The integration of artificial intelligence and machine learning into NPSLE research offers promising opportunities for biomarker discovery, diagnostic prediction, and treatment optimization. These approaches may identify complex patterns in clinical, laboratory, and imaging data that are not apparent through traditional analytical methods.
Conclusion and Clinical Implications
Neuropsychiatric systemic lupus erythematosus represents one of the most challenging manifestations of SLE, requiring sophisticated diagnostic approaches and personalized treatment strategies. Recent advances in biomarker discovery, neuroimaging, and targeted therapies have significantly enhanced our ability to diagnose and treat NPSLE effectively. However, significant challenges remain in terms of early recognition, accurate diagnosis, and optimal treatment selection.
The evolution toward personalized medicine in NPSLE reflects broader trends in autoimmune disease management, emphasizing the importance of individual patient characteristics in treatment decision-making. The integration of advanced diagnostic tools, including biomarker panels and sophisticated neuroimaging, enables more precise phenotyping of NPSLE patients and more targeted therapeutic interventions.
Future success in NPSLE management will depend on continued research into disease mechanisms, development of more specific therapeutic targets, and implementation of comprehensive care models that address the complex needs of affected patients. The ultimate goal remains the prevention of irreversible neurological damage while optimizing quality of life and functional outcomes for individuals living with this challenging manifestation of systemic lupus erythematosus.
The journey from diagnosis to personalized treatment in NPSLE exemplifies the potential of precision medicine to transform outcomes in complex autoimmune conditions. As our understanding of disease mechanisms continues to evolve and new therapeutic options become available, the prospects for improved outcomes in NPSLE patients continue to brighten, offering hope for better quality of life and long-term prognosis for those affected by this challenging condition.
 rheumahub.com
rheumahub.com